Abstract
Background: Protein kinase C (PKC) is known to regulate various aspects of platelet functions. In platelets, PKCδ is known to both positively and negatively regulate dense granule secretion in response to PAR and GPVI agonists, respectively. Hence, we speculate that the differential regulation of PKCδ in platelets is due to differences in tyrosine phosphorylation of PKCδ.
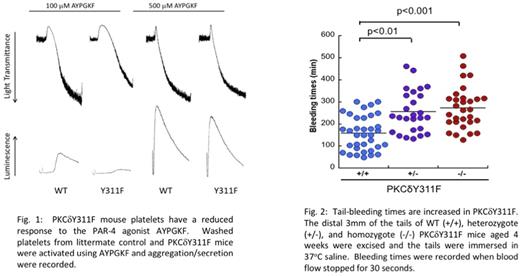
Results: We investigated several phosphorylation sites on PKCδ and found that Y311 is phosphorylated downstream of PAR agonists to a greater extent than GPVI agonists in human and murine platelets when feedback is blocked. Hence, the focus of this study is to characterize the function of PKCδ tyrosine Y311 in platelets. Therefore, we generated PKCδY311F knock-in mice to study the contribution of Y311 on PKCδ to PAR-4-mediated platelet activation. PKCδY311F mice are viable and have no gross abnormalities. Washed platelets from PKCδY311F mice have reduced aggregation and dense granule secretion after stimulation with AYPGKF, a PAR-4 agonist (Fig. 1) indicating its importance in PAR-4 mediated signaling. In an in vivo tail-bleeding assay, PKCδY311 mice have significantly enhanced tail-bleeding times compared to wild-type (WT) littermate controls (Fig. 2). Finally, PKCδY311F mice exhibit longer time to occlusion compared to WT control using a ferric chloride in vivo thrombosis model. This indicates that the phosphorylation of PKCδ Y311 is pro-thrombotic. At the molecular level, PKCδY311 was phosphorylated in Fyn-/- null platelets but not in Lyn-/- platelets by AYPGKF, indicating that Lyn phosphorylates this tyrosine residue.
Conclusions: Phosphorylation of PKCδ on Y311 is important for regulation of platelet functional responses initiated by thrombin.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal